TrialKit can support any risk-based monitoring (RBM) models that are created outside the system in analytical or biostatistics reporting. This takes Field Source Verification to a more site/subject-specific level.
TrialKit can support any risk-based monitoring (RBM) models that are created outside the system in analytical or biostatistics reporting. This takes Field Source Verification to a more site/subject-specific level, rather than applying the same rules throughout an entire study. In other words, a study manager may want Monitors to verify specific data points that need review, but those requirements may vary by site, visit, or subject.
TrialKit allows for RBM models to be implemented in a couple of different ways
-
Real-time through the TrialKit API from an external tool driving the RBM decisions
-
A built-in configuration tool to define the RBM rules
This article covers how to configure which fields require source-level verification under a known risk-based monitoring model.
Rules for what needs to be monitored are easily defined directly from the CRFs in the study. This can be done on any CRF at any site depending on what rules are being defined.
Access to configure these rules requires two unique permissions


Access to risk-based monitoring on its own will only allow users to view the existing rules. Configuring those rules and saving new ones is separate permission.
To define a new rule, open an applicable subject record that will need to be monitored.
1. Tap the workflow settings icon indicated below.
2. You will see the message "You are currently in Risk Based Monitoring Configuration Mode"
The ability to see this icon depends on access to configure risk-based monitoring from the study role security settings.
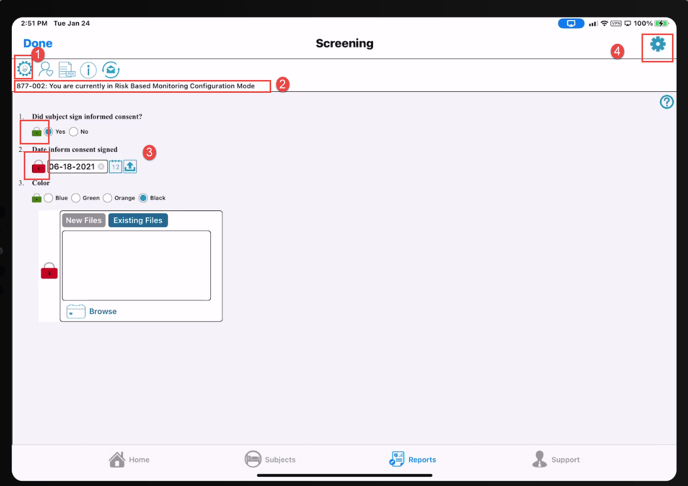
3. Tap the lock icons next to any of the fields on the form that need to be Monitored.
Green = enabled (Monitor will review)
Red = disabled (Monitor will not review)
4. Once the desired fields are set, tap the icon in the upper right corner. This is where the rule will be defined and saved.
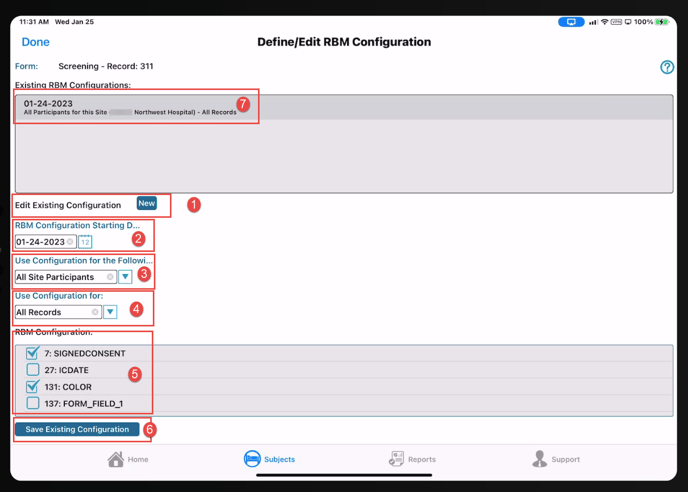
The starting date for the rule is compared to the visit/transaction date on the form, not the current date when Monitoring takes place.
- All participants in the study
- All participants at the current site only
- Just the participant currently open
- All visits where the current form is collected
- Only the record that is currently open
4. Use configuration for - Choices are following
- Just this record
- All records
5. RBM Configuration
- Checked fields are FLSV enabled
6. Be sure to save the configuration
7. To edit an existing rule, tap it in the Existing configurations table and change any of the parameters listed above. Then tap Save.
