Managing Data Related to Users, Sites, and Studies
TrialKit has always provided a solution for collecting data at various levels (User, Site, Study, and Subject). Read more here about how to create forms to collect this type of information.
The CTMS Center joins all non-subject data collection into a single area for viewing and reporting.
Accessing the CTMS Center
Prerequisite
The user's host-level role has Access to CTMS
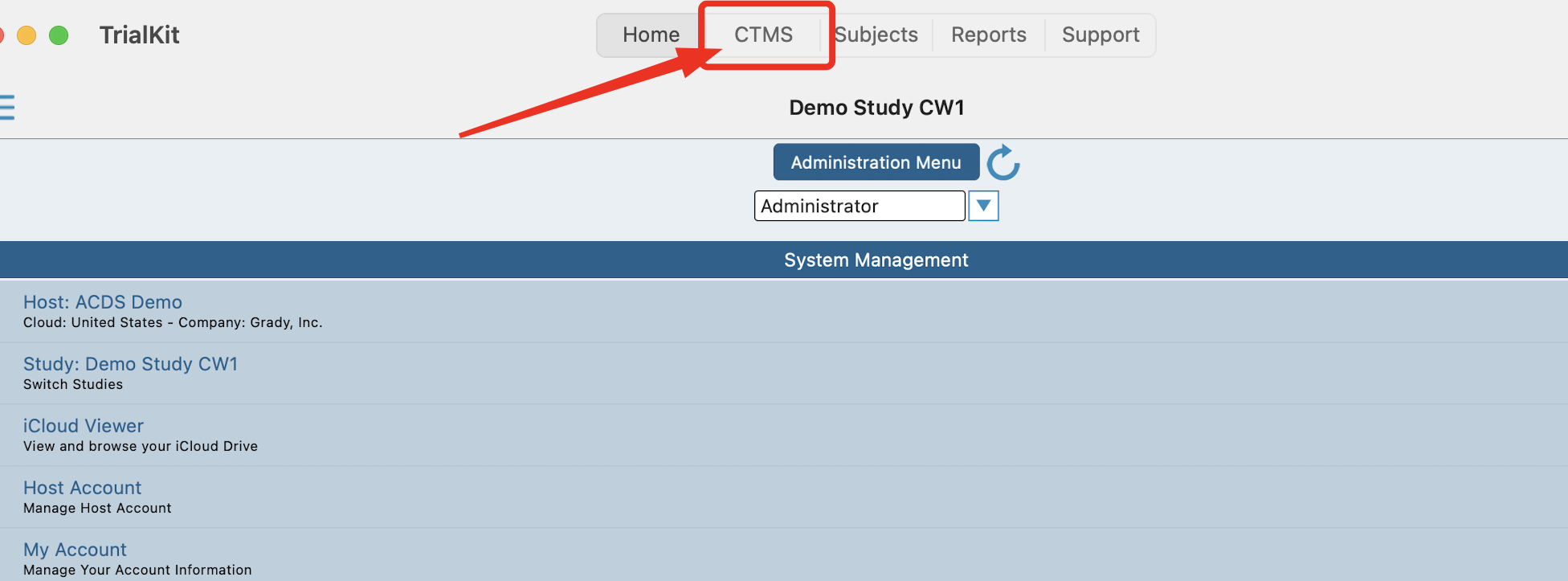
Web Browser:
Located under the Host menu
.png)
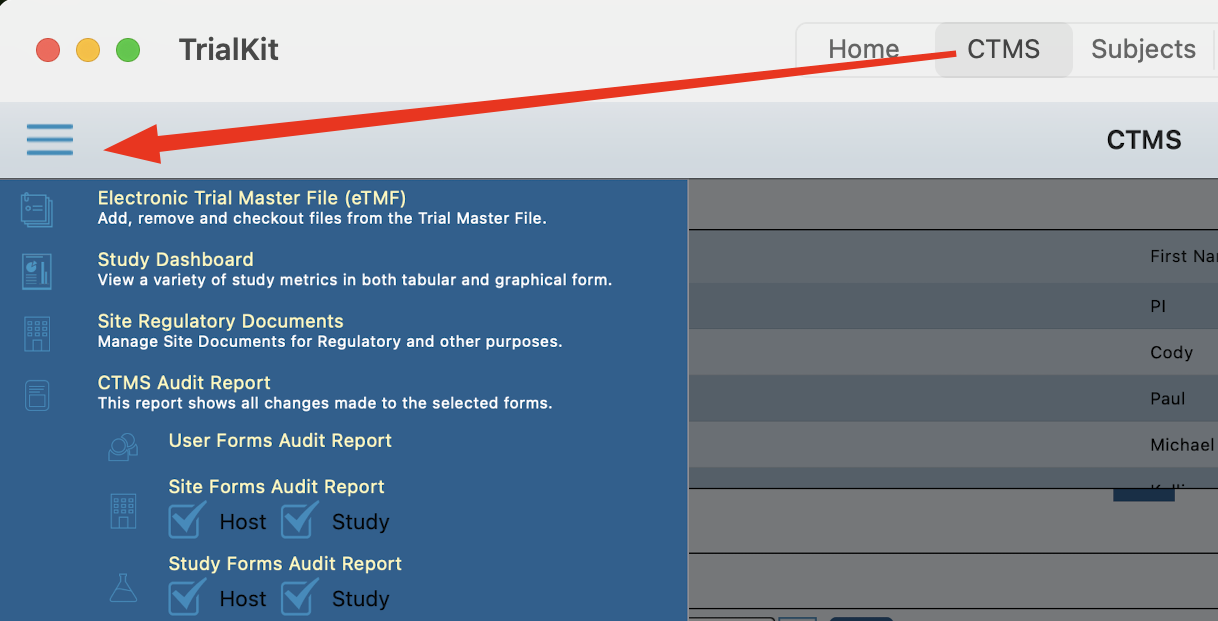
App:
Located in the main navigation bar. This may be either at the top or bottom of the screen depending on which device is being used.
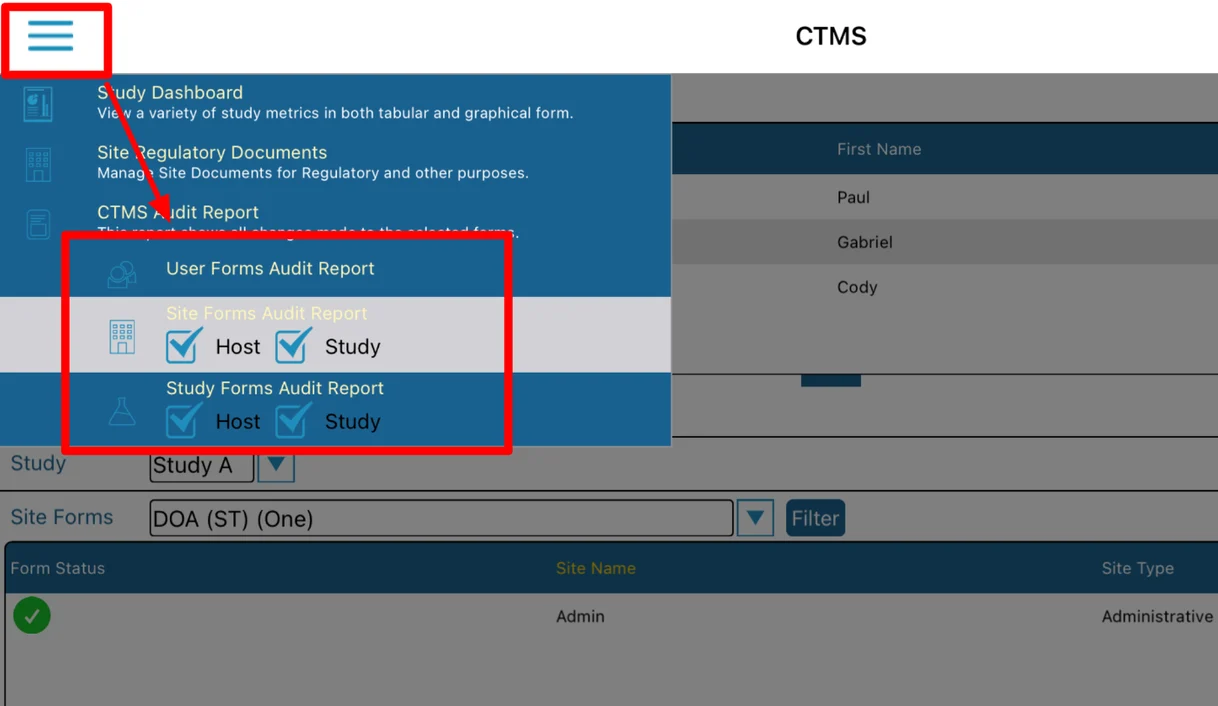
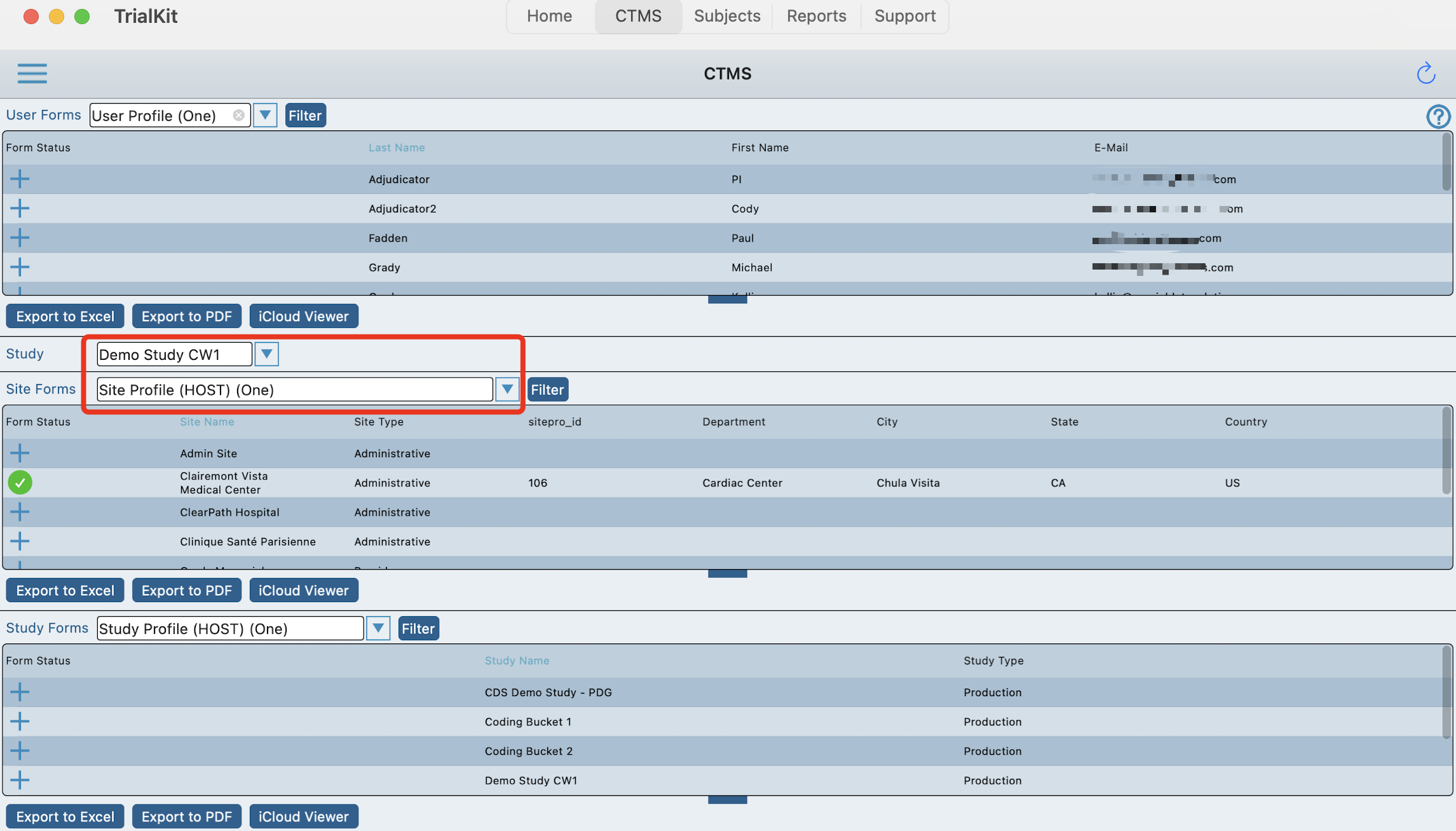
There are three possible sections, depending on the viewing user's form rights:
User level information
Site level information
Study level information
Additionally site and study type forms are further broken down into Host level and Study level. For example, a study level site form might be something like IRB Approval information because it's specific to each site in a given study. In parallel, a host level site form might be something like Site Contact information because it's specific to a site, but does not change from one study to another.
That level of granularity requires a user to select which study they are currently viewing information for as shown below.
Caution
If the study selection is changed, that will change the location of the current user. In other words, if you exit the CTMS and open the Subject Manager, the subjects will be displayed for the study that was last selected in the CTMS.
Navigating the Tables
The columns and details displayed in the tables are based on what has been configured by an Administrator. That is discussed in the next section below.
There is potential for a large amount of information in a small view, so each table can be adjusted as needed with the notch at the bottom of each table as shown below.
Tapping any record will open the form to see the full details or add/edit, depending on permissions.
.png)
Note
The CTMS Center on iOS is also where the current study's Dashboard and the host's Trial Master File can be accessed (via the menu at the upper left)
Configuring the CTMS
By default there are no steps require beyond simply giving users access to the CTMS. This will provide access to all the forms and information needed. However, those tables can be set up to include more information without requiring users to open up each record.
Those table columns must be pre-defined within the form builder, via the field property shown below.
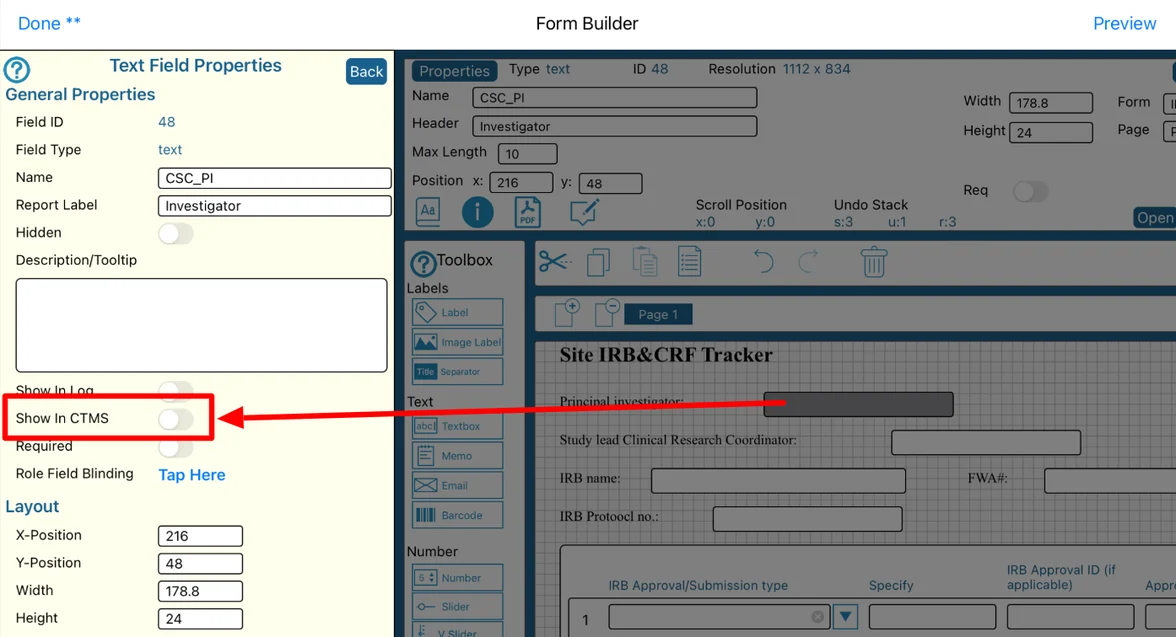
By including the field in the CTMS, in the example above, when a user is viewing the Site IRB Tracker in the CTMS, they will readily be able to see the name of the Investigator. Otherwise, they would need to open each record to view that information.
Site, Study, and User Audit Reports
Activities on data within the CTMS are all logged by the system. These can be exported on a per form type basis. Note, activities prior to April 16, 2023 on non-subject data will not display in these audit reports.
Prerequisite: User has host level access to the CTMS Form Audit Reports
Web Browser:
Within the CTMS, the export links are located on the right side of the screen.
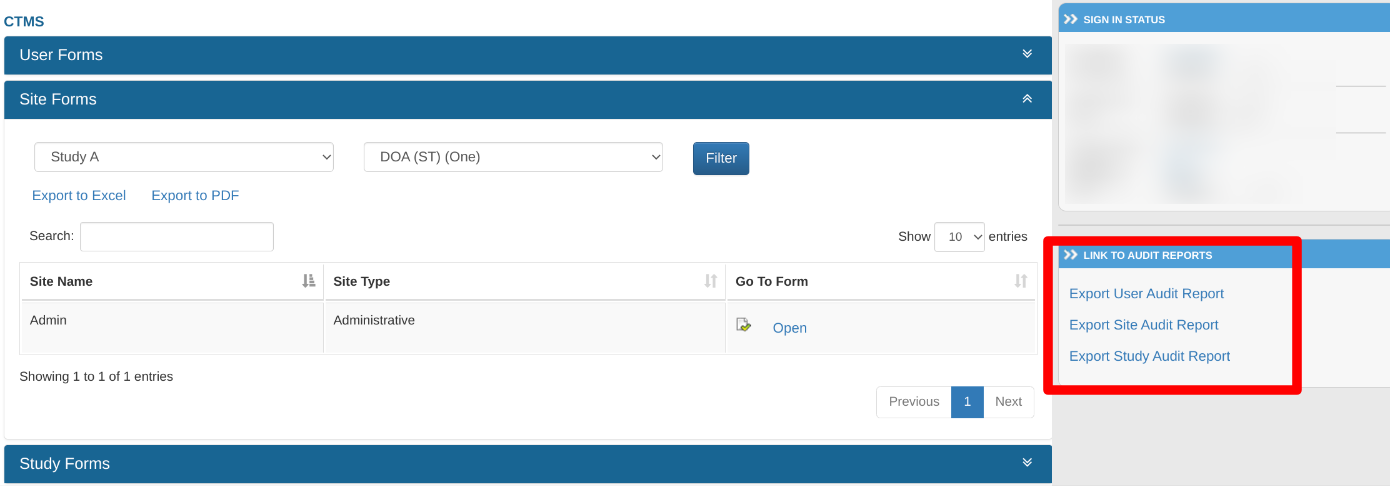
App:
From the CTMS screen, tap the menu at the upper left to access the audit report options. Then tap on the desired form type and the level of data to include (Site and/or Host levels) as shown below.