How Diary Schedules Work
Tip
This article expands on concepts initially covered in the article on configuring epro studies to build a full understanding of the flexibility in ePRO form deployment options. It's recommended to understand fundamentals before reading this article.
Diaries are the recommended method for delivering surveys or forms to Participants as action items to complete. A diary schedule is simply a diary configuration consisting of one or more forms that need to be deployed to Participants at a specific time interval or on specific days of the week for a pre-defined period of time. For example, a survey that needs to be completed everyday for 7 days prior to the 1 month follow up visit interval.
How a diary schedule in a study is applied in a study:
Based on how a diary is configured, when the defined diary trigger date is saved for a Participant, TrialKit builds and stores the diary schedule(s) behind the scenes. This is a timeline table of all the forms that will need to be deployed to that specific Participant.
Here are a few points to be aware of:
The timepoints of the schedule will be based on the Participant’s timezone at the point of the diary being triggered. If the Participant’s timezone changes later, this will update their scheduled timepoints +/- the number of hours that their timezone changed.
If a diary is triggered multiple times, it will be scheduled based off of the record with the most recent visit date unless the trigger date is blank.
If the subject is not a Participant user in TrialKit and a diary schedule is triggered to be built, the trigger form will receive an error. This is the system telling you it cannot build a schedule for a Participant user who does not exist.
Viewing Diary schedules can be done at the subject level, for the whole site, or the entire study.
Diary Schedules For a Specific Subject
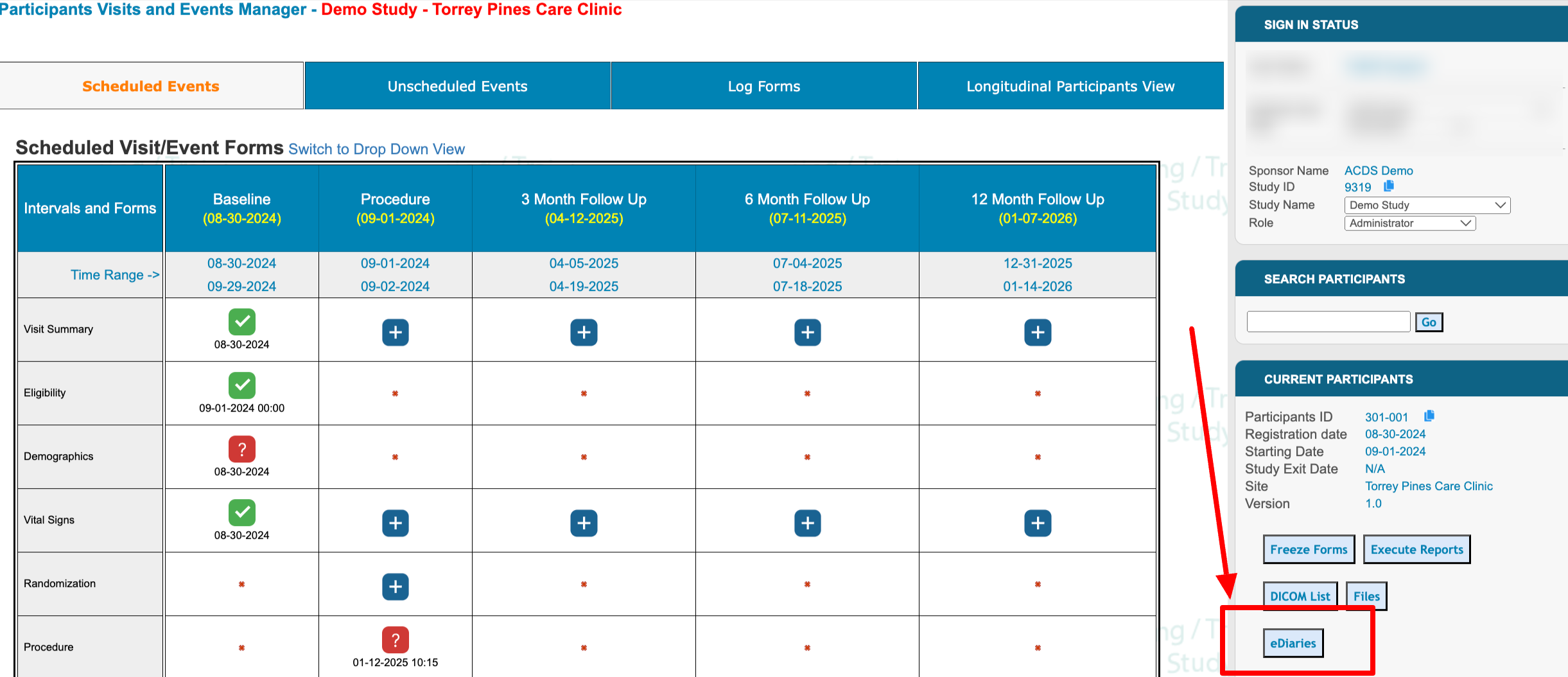
The diary schedules can be accessed within each subject's casebook.
Web Browser:
Mobile App:
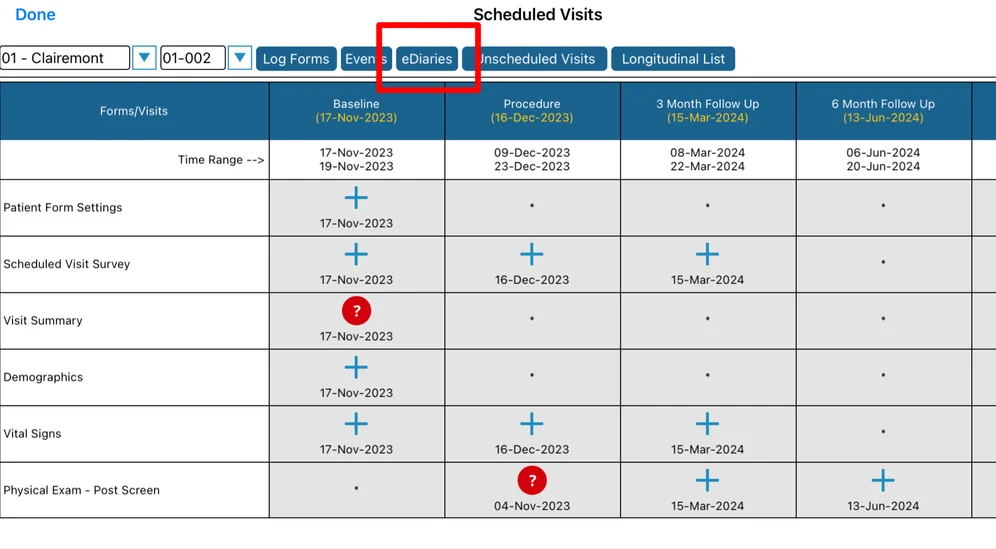
Within this report, a specific diary can be selected above the table to view the corresponding schedule, if one exists for the current Participant.
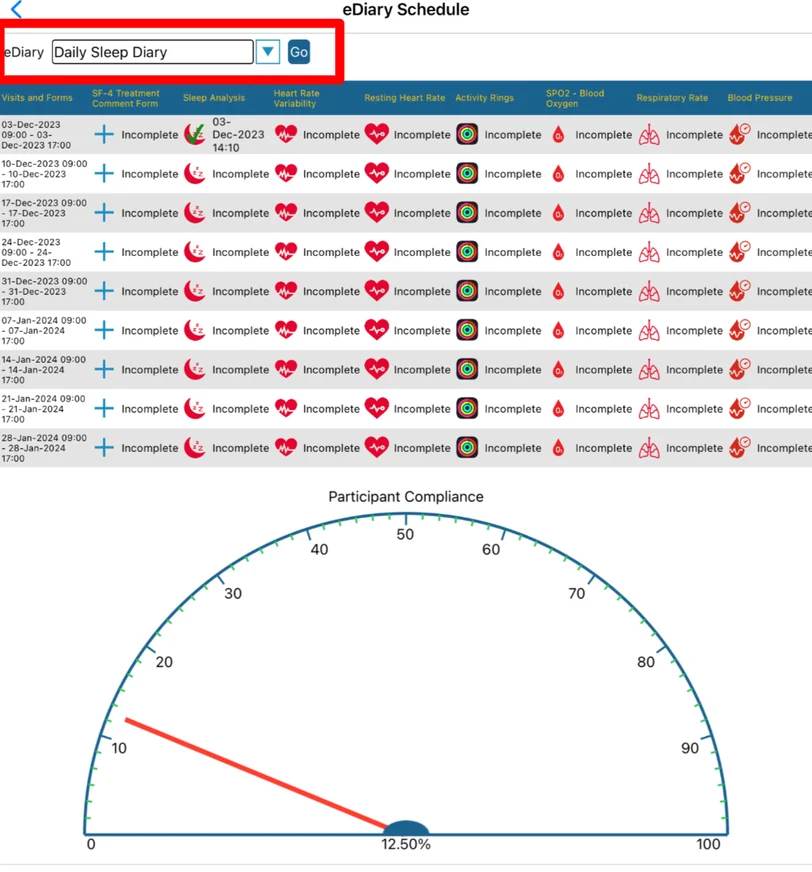
As shown above, the purpose of this table is to display what is expected of a given diary schedule for that Participant and how well they have been keeping up with their form submissions over time (diary compliance)
Note
A diary schedule is different from an event schedule. Event schedules are intended for use in event follow up scenarios, where the "event" is something that the Participant will trigger at any time. Read more here about event schedules.
When the Diary Schedule is not Created
There are a few scenarios where the timeline cannot be built:
The diary frequency is set as zero, or always available.
The Participant's study exit form has been entered with a date that precedes the diary start.
The form that starts the diary is not yet known. For example, if a diary is set to start when a date on the screening form is saved, but that date has not yet been entered. This means TrialKit cannot build the timeline for that diary until that date is saved.
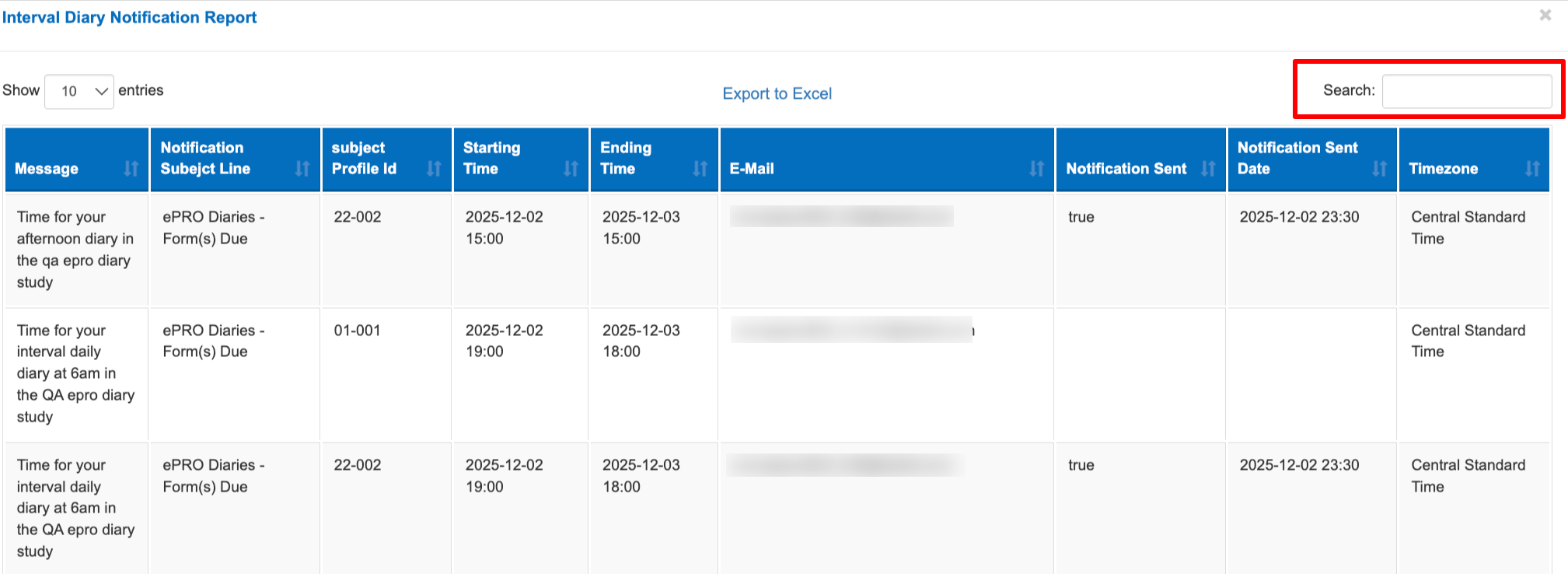
Interval Diary Notifications Report
Since the diary schedules drive notifications and what is due at given points in time, this is a helpful report to reference the existing schedule that the system has built in the background when the diary was triggered. In most cases this would match the Diary report above, but there are a couple instances where it could differ:
The diary configuration rules have changed since the schedule was last triggered/created
The Participant changed timezones since the diary was triggered
If a Participant is missing many of their forms, it could be due to notification reminders not being received. This report will show if TrialKit has an existing schedule of notifications going to that Participant, and show if one was sent recently.
Access is dependent on user having permissions for eDiaries > Interval Diary Notifications Report
Web browser:
The Interval diary notifications report is located within the diary compliance report.
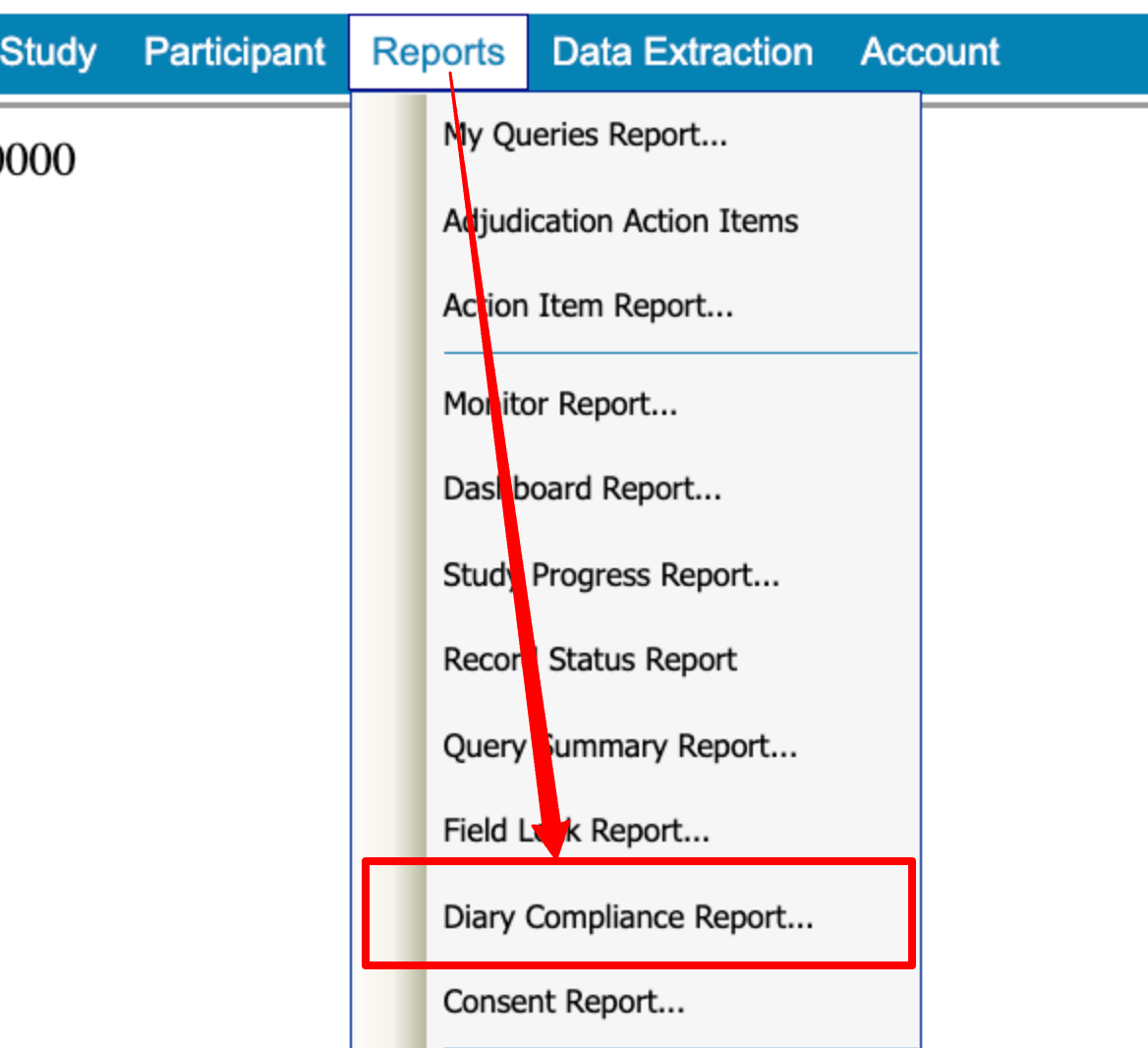

From this report, use the search box to look up a participant ID or email to see the full
App:
Events that can cause an existing interval diary schedule to get cleared
Saving a stop date on some other form
Resaving the trigger form without a trigger date (ie. date is blank. Read here)
Subject Withdrew/exited from the study
Had a moving average termination
Site Status was deactivated
Study status is set to locked or completed
Diary Compliance
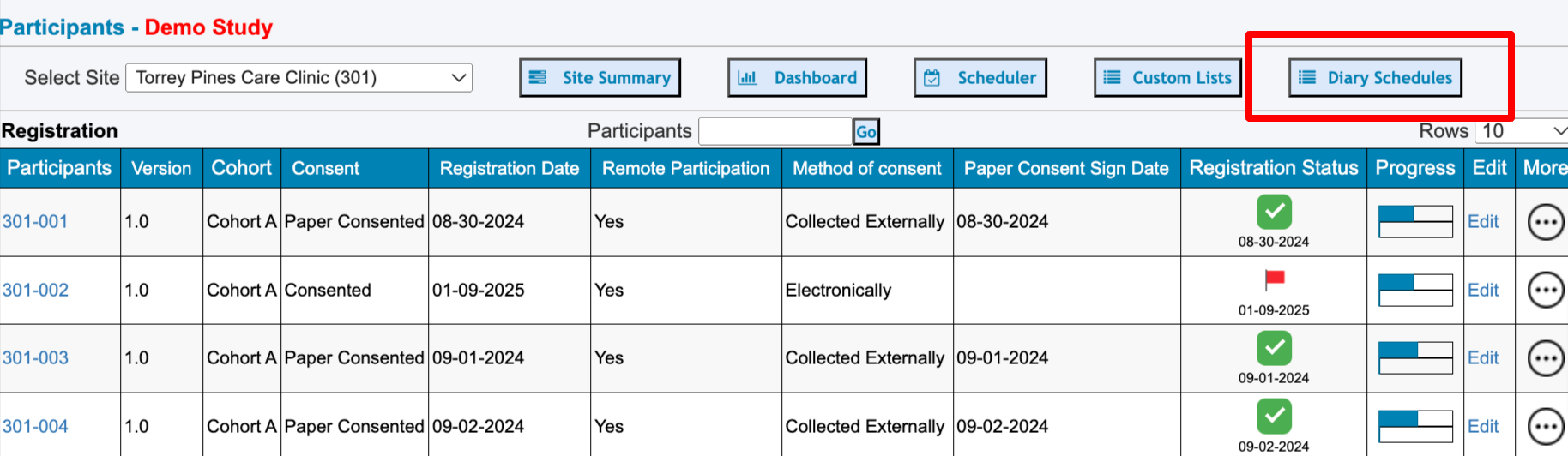
Diary Schedules For All Subjects at a Site
This is helpful for Site Staff to see which Participants at their own site are keeping up with planned diaries. It also allows for viewing upcoming expiration dates in the case that contact needs to be made to maintain compliance.
From the Subject Manger screen, the Diary Schedules for the current site can be accessed:
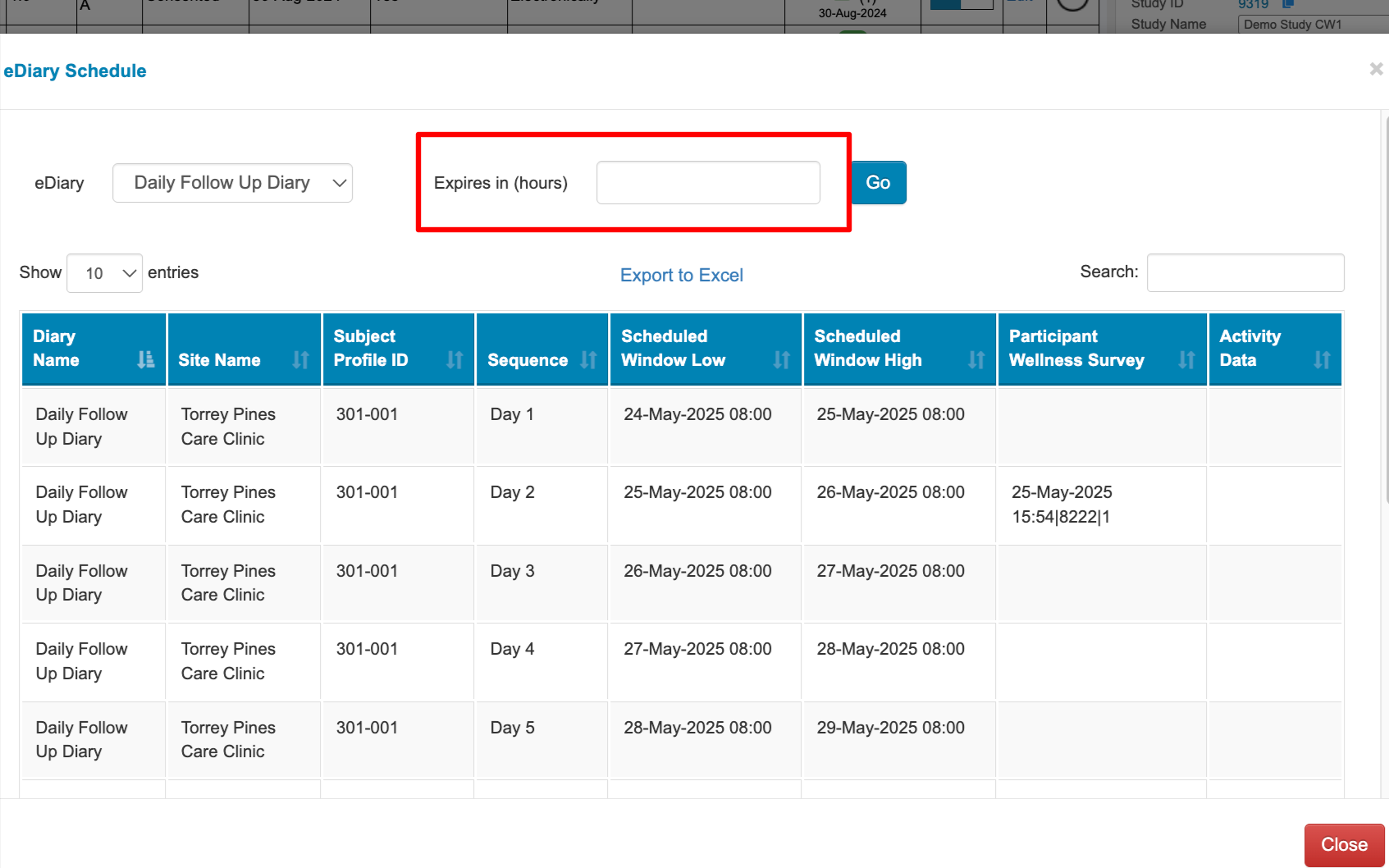
The resulting report give a list of each scheduled diary window. If the diary was completed, the date and record ID will be listed in the right column.
An expiration value (hours) can optionally be entered to filter for all subjects that have diaries that will expire within the designated window.
For example, entering “24” will display only the subjects/diaries that will be expiring within the next 24 hours. It also accepts negative hours. “-48” will return any windows that expired 48 hours prior to the current time.
TIP
Use the expiration filter to help stay in touch with Participants that appear to be falling behind
Diary Schedules For The Entire Study
A table of diary compliance can be viewed across the entire study, or by Site
Prerequisites
User must have permissions granted for access to eDiaries>eDiary compliance report
Web Browser:
Found under the Reports menu
Mobile App:
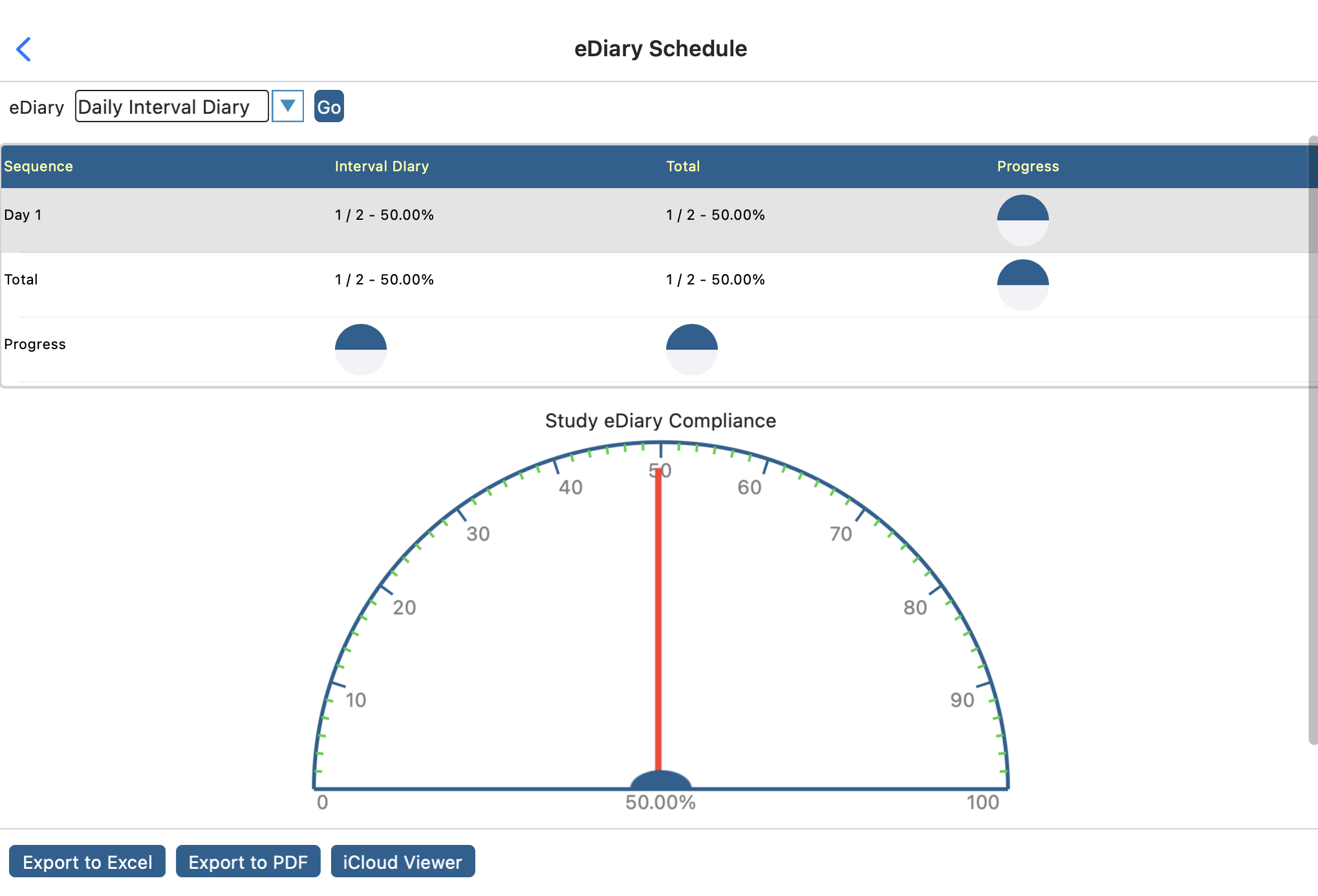
In this very simple example, a 1-day diary is scheduled for each subject. There are only 2 subjects in the study where one of them has completed the Day 1 diary within the expected window. Therefore, the compliance is at 50% for the study.
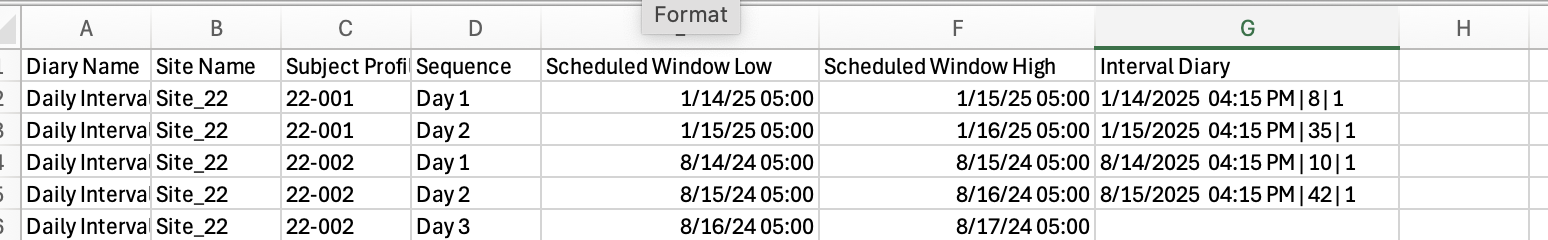
From the web, the same report can be exported with additional details about each diary interval and when it was completed.
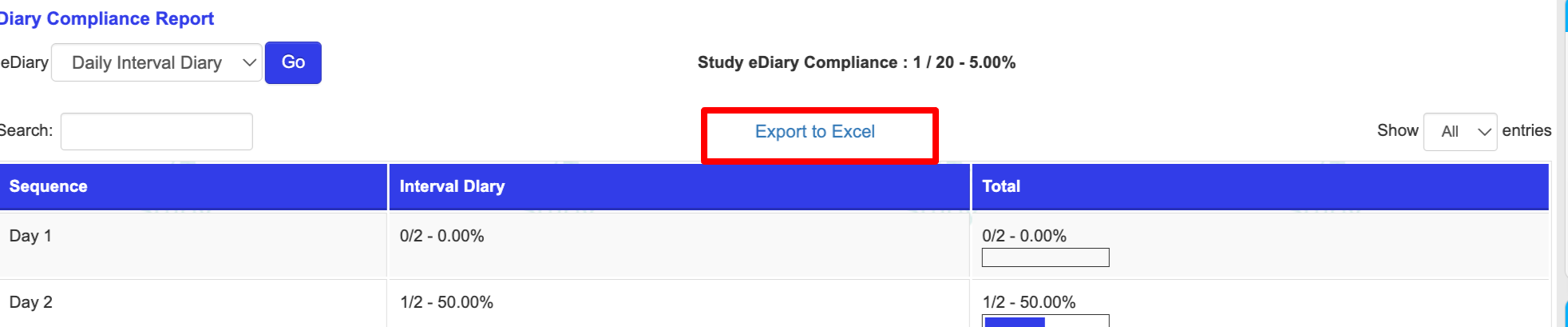
The exported file includes the low and high window of each interval and the date that each form was completed. In this example below, one form is collected for 2-days. The date of completion in the last column also includes the record ID and status of the form (DateEntered | RecordID | RecordStatus )
Troubleshooting Reports of Diary Unavailability
When Participants report any unavailability of their diaries, or alleged access problems. There are a few steps that can be taken to confirm if the issue is user error or if there is a problem with the diary schedule.
Start with checking the Participant’s diary schedule to determine when their window of availability is expected
Double confirmation of the notification schedule can be made by the study Administrator. This verifies that the current schedule matches the notifications being sent to the Participant.
If both of the above are correct, then verify if the Participant logged in at the correct time via the sign in audit. This is normally limited to Administrator users.
Any inconsistencies with the above reports or unexpected behaviors should be reported to TrialKit technical support by the study management team responsible for the study’s configurations and knowledge of intended diary requirements.