Obtaining Consent From a Study Participant
TrialKit has the capability of tracking consent for study subjects or registry participants. This article covers how Site staff can use TrialKit to consent participants, and if applicable, access a partially signed consent form to apply additional signatures.
Consent Basics
Prerequisite: Study is configured for ePRO and consent by an Administrator
If a study is tracking participant consent, it will be shown directly in the subject list within the Subject Manager by a consented/not-consented indicator next to each subject.
Tapping on a subject that is not consented will prevent opening the casebook. This is a visual on the Mobile App:

There are three ways for a participant to provide consent:
- Self-Consent
- In-person on site
- Paper consent
Self-Consent
If the participant’s email has been entered on the registration form, the participant will have direct access to TrialKit via the Web or Mobile App. They will be able to sign in and access their consent form. Once that's completed, their subject ID will show "Consented" as shown above.
Read more here about how Participants access and complete consent directly.
In-Person on Site
If a subject is on-site, tap the Handoff button in the upper right corner to enter handoff mode, select the desired subject in the resulting list, and handoff to the subject.
Mobile App:

Paper Consent
The system allows for participants to bypass electronic consent and use an external process - typically paper consent.
If this function is allowed on the study, the participant’s registration form will contain a checkbox to indicate the use of paper consent.

Adding Investigator or Other Clinician Signatures
Often times the consent form needs to collect more signatures than only the Participant's. In these cases, the signatures on the form will be assigned to the necessary user roles. For example, the site Investigator may be required to sign after the Participant signs.
Consent forms are accessible within the subject casebooks under the log forms - but only if the subject is fully consented (i.e. all signatures are collected). If signatures are missing on consent, the subject casebook is not accessible. Because of that, the consent form must be accessed from the main Subject Manager screen.
Prerequisites: The user has access to Signature by Role Management, and is assigned the role responsible for countersigning a signature on the consent form.
On the Web Browser, tap on the subject ID. If the subject has not started their consent, a window will indicate the following:
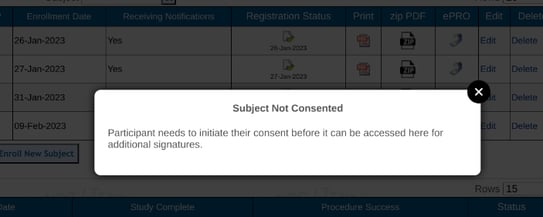
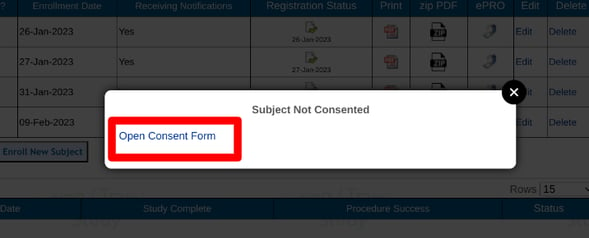
If a consent record does exist, but the consent is not fully completed, there will be an option to access the partially completed form as shown below.
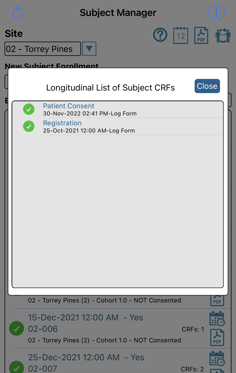
On the Mobile App, tap and hold for 1 second on the desired subject ID. This is a shortcut to open the full list of existing records for the subject. Tap the applicable consent record to open the form and sign it.
Required Signatures by Role Report
The Signatures report is a list of all forms and expected/applied signatures on any signature fields that have been assigned to a user role by a study Builder.
Prerequisite: User role has access to Signature by Role Management
This report is helpful as a single place to see where signatures are missing, or to apply signatures in batch to multiple forms.
For example, as a Site Investigator, the report could be opened at any time to catch up on the subjects awaiting signatures on their consent forms in order to be fully consented in the study.
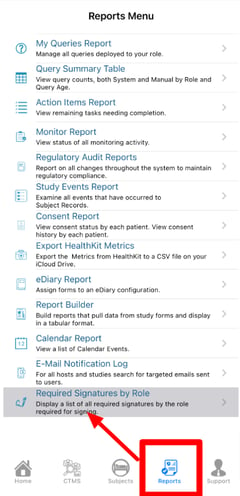
To access it, currently only via the Mobile App, open the Reports menu as shown below.
Reconsenting a Participant
Sometimes it is necessary to have a Participant re-consent. To do this, they must first have their previous consent revoked. That is done either Administratively or directly by the Participant if the study settings allow for it. Doing so will require the Participant to fill out consent again before any further data entry.
If the Participant is consenting on a new study version, where they have already consented on the previous version, the new consent form will only collect the Participant's signature - assuming the signature is assigned to that role. Any potential counter-signers (Clinicians) will not need to re-sign subsequent versions if their counter signature already exists in the previous study version. If it was just added in the new version, the Clinician signature will be required to fully consent.
If a Participant is consented currently, and the consent record is deleted, the Participant will no longer be consented. This will be the case even if multiple consent records exist. Any one of the records being deleted will cause the Participant to lose their consented status.
