TrialKit v7.15 - October 2025
This release brings several highlighted features described below and many other improvements and bug fixes. Scroll to the bottom for the exportable full release notes.
All applications rolled out by October 12, 2025
Note, this release documentation includes changes of some already-released recent patch updates during the previous month.
Mixed label format editing when using the app form builder
Based on popular request, the label object now allows for applying different font styles to words within a single block of text. It is also supported within forms on mobile if the form was built using the web form builder. Read more.
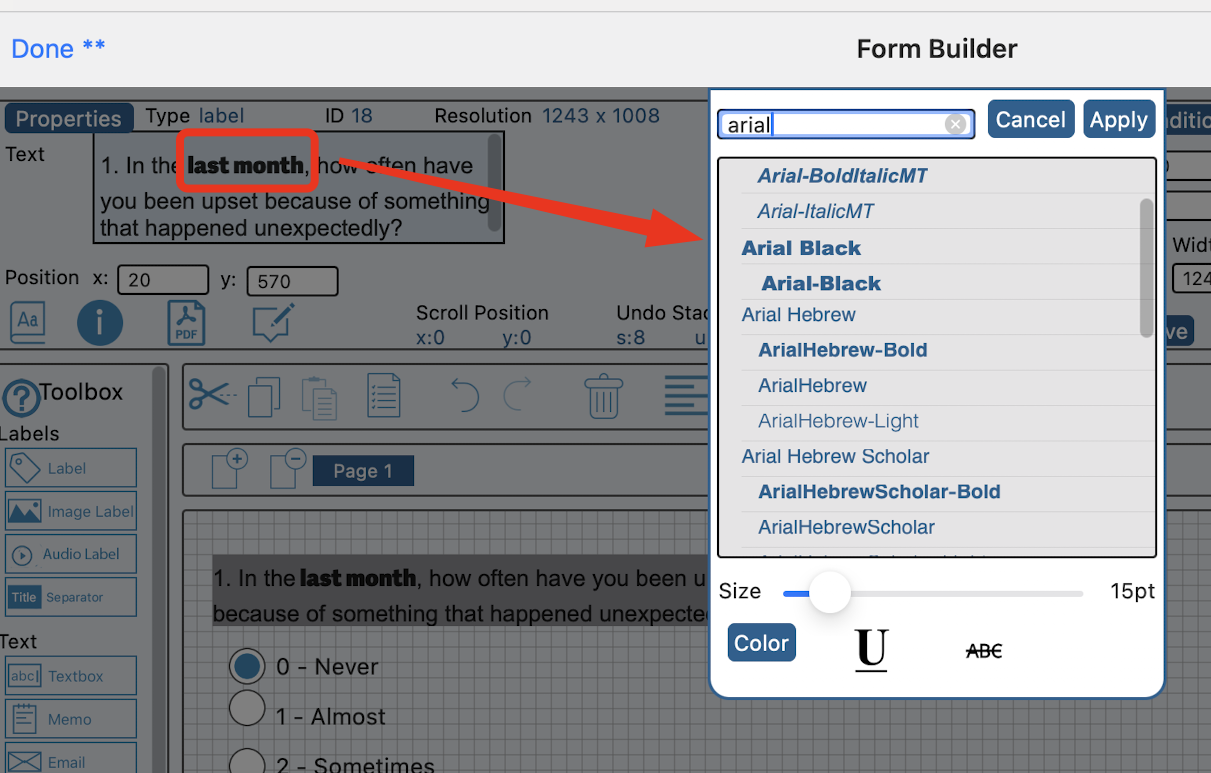
Enhanced Participant Notifications Report
Notifications can now be more comprehensively tracked and used for sending manual notifications to Participants. Read more.
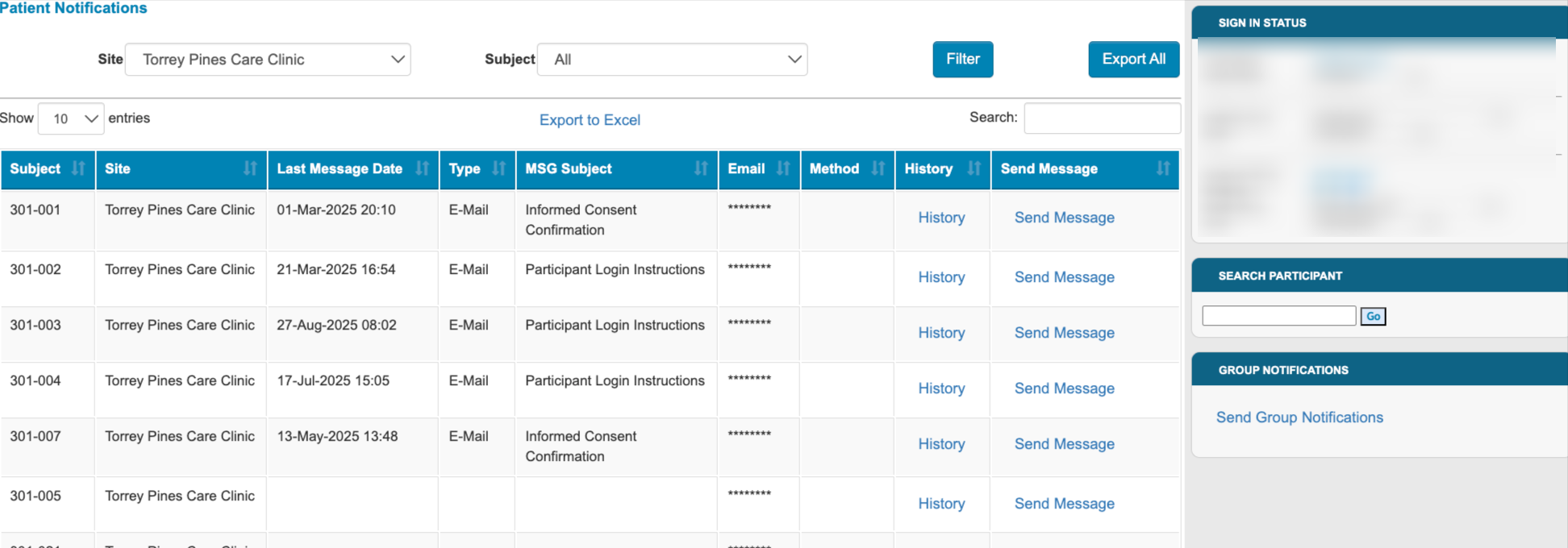
Randomization Totals
Studies that use the randomization utility can now allow site users to view totals. This is based on a new permission.
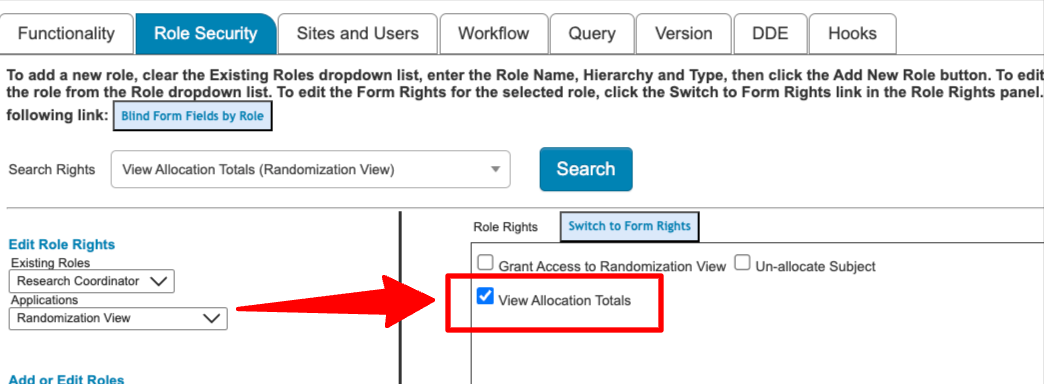
Configure Study Calendar Reminder
Site-Participant scheduling will now send pre-defined reminders to the assigned Clinician and the Participant prior to the event. Read more.
Consent PDF Improvements
The PDF that Participants receive via email has been simplified and formatted to more closely mimic how it is presented on screen. Additionally, Participants can export the same PDF at any time from within the web portal. Read more.
Capture Images and Videos from the web
Image and Video fields on a form previously only allowed camera access from mobile. Now images can be captured on the web as well with the front-facing device camera. Read more.
Diary Stop Dates Made More Flexible
Now a diary stop date can be defined on any form. Previously it needed to be a computed date on the same form that triggered the diary. Read more.
Other Worthy Mentions
PDFs now support hidden tabs in data view format
DICOM uploads now write the subject ID to the dicom data to improve organization within PACS
Consent date now has the ability to automatically update the registration date. This will allow the visit schedule to be built from date of consent.
UI improvements to the Participant web portal to better support mobile web browsers for Participants that are not using the app.
Download the full release notes ⬇️
TIP: Download the release notes below and use the embedded knowledge base links to learn about each change
Note: The final release notes are subject to minor changes up until the date of the release
Version Certificate
Crucial Data Solutions provides a summary certificate documenting the assessment and procedures followed for each new version corresponding to the changes documented in the release notes.
Impact Considerations
Impact ratings can be found within the release notes for each change. Any changes that have potential to impact study configurations, expected existing behavior, or changes to common user navigation will be highlighted here.
Dicom File uploading now writes over the patient ID in the dicom tag data
PDF exports in data view format now exclude conditionally hidden tabs
Consent PDF that gets emailed to the Participant is now formatted differently