What is web version 7.0 and what it means to your study
Version 7.0 is the next major version of TrialKit that is getting released in the fourth quarter of 2023. It brings several great enhancements to TrialKit - with a focus on forms experience, modernized icons, and graphical reporting.
Here's a quick look at a sample of a home screen where you will immediately notice some changes, but nothing that will require your users to be retrained. All the mechanics of navigation, data entry and data review will remain unchanged.
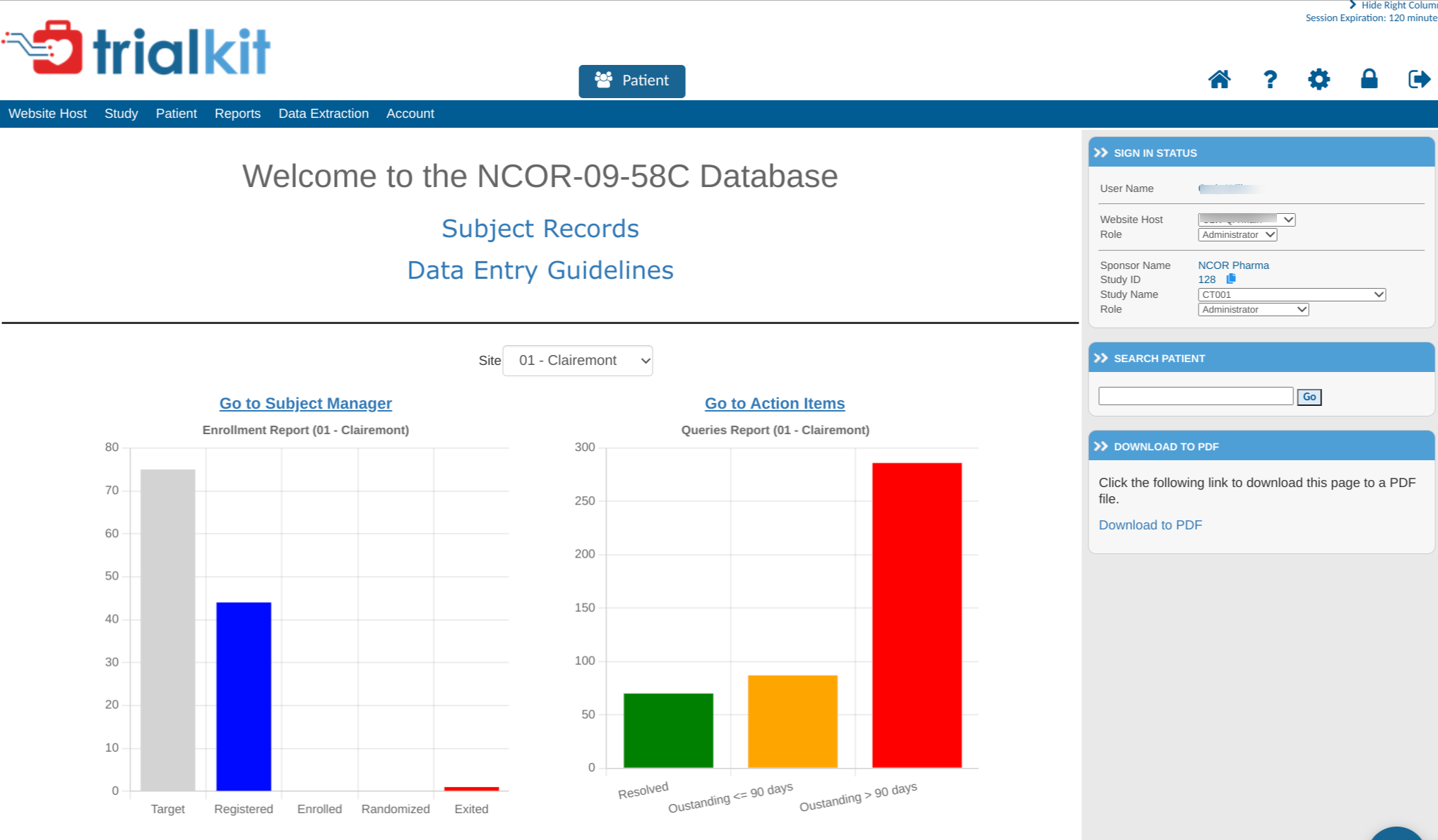
Version 6 vs Version 7
Version 6 has been around for a couple of years, going through several minor version updates, each that have brought a handful of new features and bug fixes to keep TrialKit running smoothly and effectively for its users.
The fourth quarter of 2023 will end on TrialKit web v6.22 and update it to the next major version - 7.0 for all users. This will not require a migration, and study Administrators will not need to take any immediate actions.
Like any version update, it's important to consider what the determining factors of a new major version are, in order to align your internal processes with the changes that are being released.
These two factors are what determined that TrialKit will be up-versioned to 7.0.
- Multiple parallel changes to a part of the system that involve saving of data records
- High volume of changes that cumulatively increase inherent risk of impacting existing functions
Risk Rating
The risk has been rated Low based on the scope of changes and the method in which the higher impact changes are being made available. More information regarding that assessment and the QA-driven process followed as part of any major release will be available in the version certificate issued prior to the update.
A major version number (7.0) means TrialKit has received a full QA re-certification that lines up with CDS's development practices. This helps CDS ensure that all of the changes being implemented do not impact any of the functions that you rely on for your current studies. That certificate is available upon request after version 7.0 is released.
TrialKit web v7 Includes the Following Changes For All users
These are the low impact changes that are typical of a typical minor release and will be immediately available in all studies.
- Several new enhancements to improve the user experience and add new functions, including some minor design improvements and common site reports like the new Site Summary, the Dashboard report, and the native home screen reports.
- Better filtering in the Action Items report, forms with open queries
- The ability to redeploy queries to new roles in batches
- Many other changes worth reviewing are detailed in the release notes (available late October 2023)
High Impact Changes Included in v7 That Will Not Be Forced
Study Admins can enable these on a per-study basis once they have had a chance to evaluate the changes.
- An all new and modernized version of forms to make data entry easier and improve greatly on the speed of form dynamics. This is the highest risk change in web version 7.0, which CDS recommends as the basis of performing validation of any study that will move to this format.
In addition to other updated buttons included in v7.0, it will come with some changes to form status indicators:
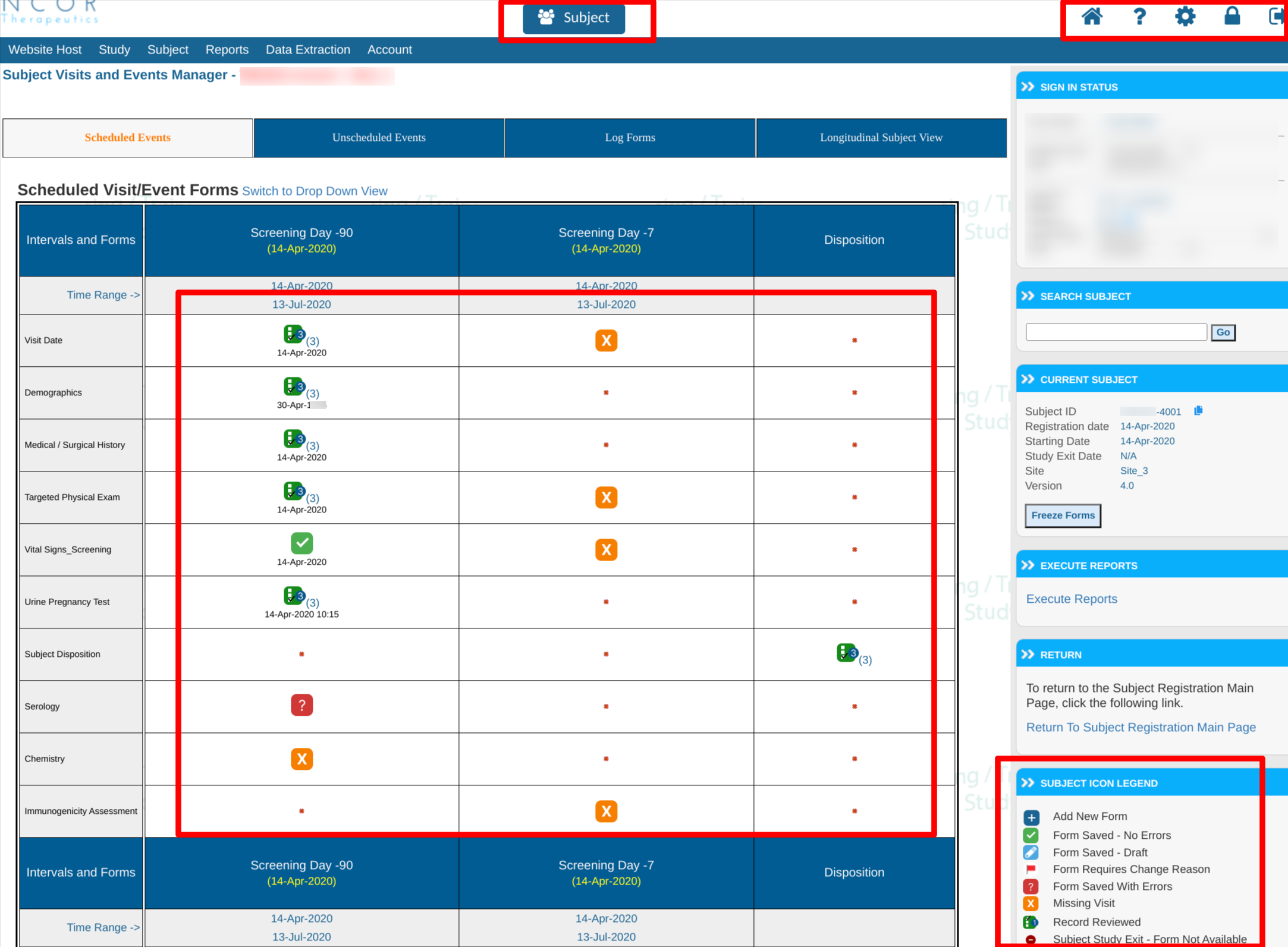
Other Changes That Will Not Be Forced
These are changes that have no impact on studies, but must be enabled by a study Administrator before they are available
- Native home screen reports to display key metrics to the site users
- Participant access to payments (on applicable studies)
What This Means For the Future
Forms and data entry are a key component of the TrialKit platform. CDS recognizes the impact involved in modifying that part of the system for any existing studies that have already been validated in TrialKit version 6.
Based on that, CDS will retain and continue full support of the existing legacy web forms through the end of 2024. At that time, a migration plan will be implemented to update all studies to the next-generation of web forms.
Here are three scenarios to help you determine the best course of action on your studies:
- Studies that are planned to end in 2024 won't need to have anything done. These studies can continue to run on the legacy forms without expecting any surprise changes.
- Studies that are planned to begin December 2023 or after should consider enabling the next-generation web forms and performing validation in that environment.
- Already-existing studies (prior to November 2023) that will continue beyond 2024 should begin putting a validation plan in place to validate the study on the new web forms. CDS will help with this process in two ways:
- Provide a validation template and instructions (to be posted at a later date in this article).
- Upon request, CDS will provide a copy of any existing live study, with data, for customers to use for validating the next-generation forms experience.
Please contact Support if you have any other questions about this version update.
