TrialKit v7.8 - November 2024 - Release Summary
This release brings a dozen highlighted features described below and several other improvements and bug fixes. Scroll to the bottom for the exportable full release notes.
Rollout completion by December 1, 2024 for Web v7.8, iOS v11.2, and Patient Web v2.12
December 21, 2024 - Coder v5.1
New Feature Highlights
Form Linking 🔗
A new form building object “Form Link” is now available on both web and mobile form builders. This allows for mapping the form to another child form. When users enter a form from its parent, the two records are tied together and the relationship can be seen via a new variable (Related Log TransID) in both the report builder and data exports.
New Types of External Variables
Two new types are being introduced:
Any Record - This lets study Builders create conditional logic that can reference any data in a given variable, such as a value that repeats over time
Parent - Applicable to the new linked forms feature, where a child form can reference a variable on its respective parent form
Form Index in Subject Casebooks 🗂️
The next generation forms experience on the web is introducing a new way to navigate quickly to other forms. This is done by a new icon found at the top of any form. Any studies that have not yet enabled the next-gen forms are encouraged to do so by March 2025.
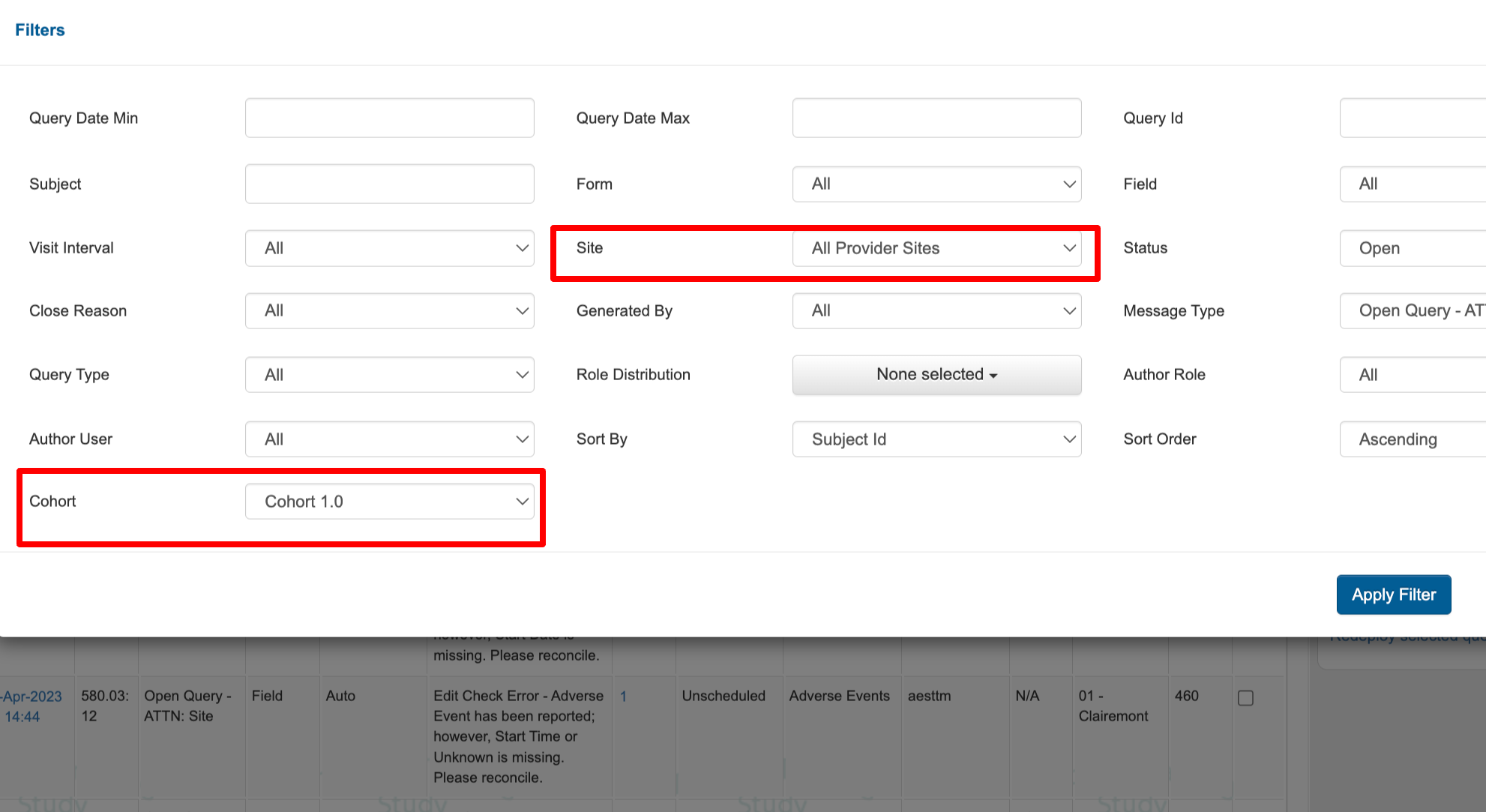
Added Filtering Options in various reports 📊
The Queries Report and Monitor Report can now filter by visit cohort and All provider sites
The Field lock report can now filter by visit and All provider sites
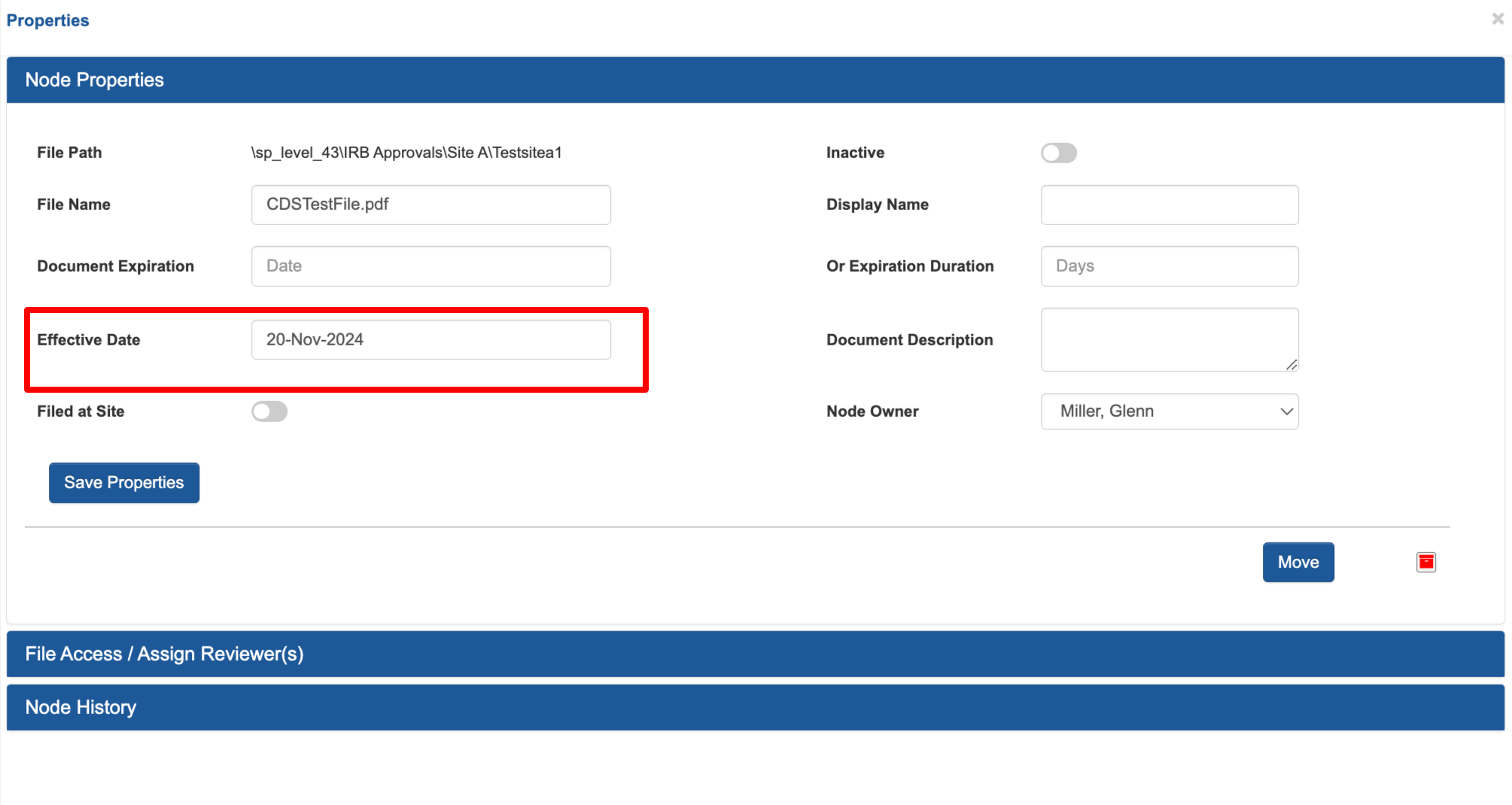
Trial Master File Effective Dates 🕑
Effective date is a new property available for files. It gets set automatically when a file is uploaded, but can be manually changed at any point. There have also been some usability improvements when navigating the file properties and performing actions in the main TMF table.
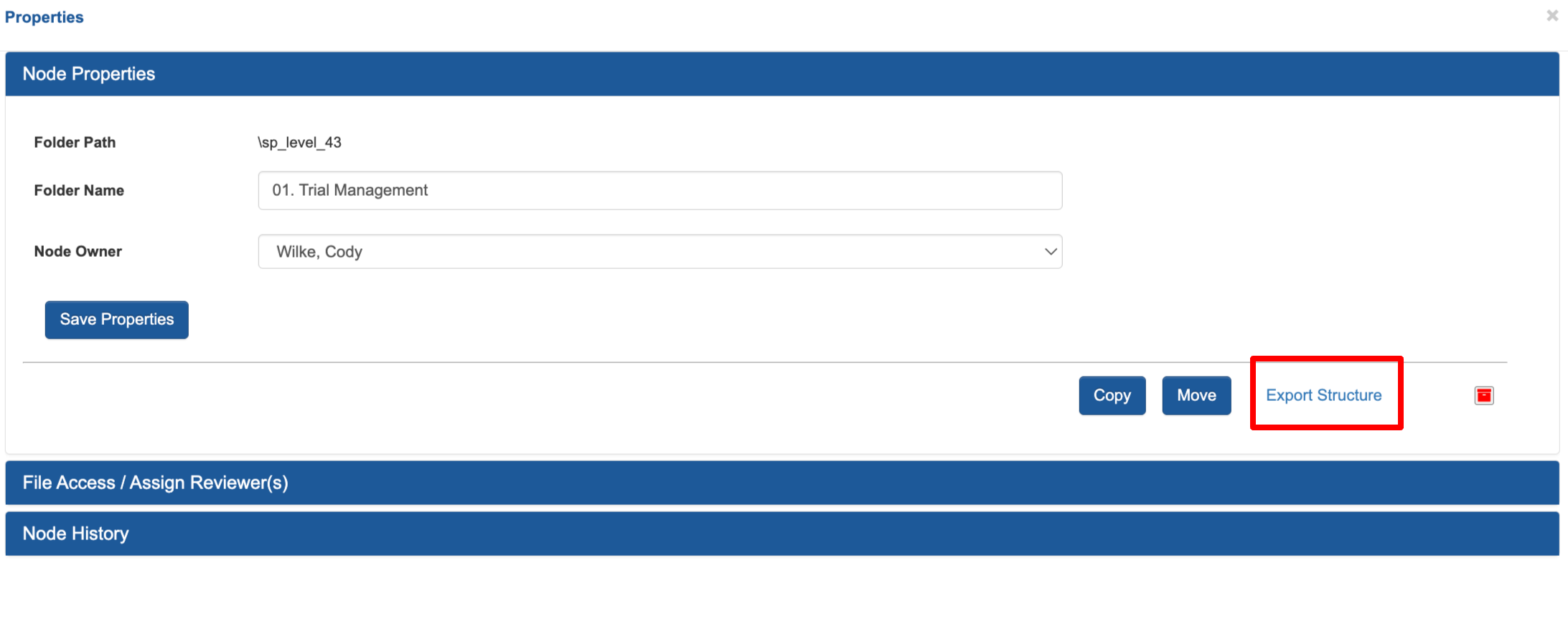
Trial Master File Folder Structure Export
The folder structure export is highly improved with more details broken apart to make analysis and audits easier. That is accessible at the folder level, or for the entire TMF. Shown here from the folder level:
PDF Format Improvements
When exporting form PDFs from either the form builder or the Subject Manager, form headers no longer get repeated on each page. That information is now displayed in the footer area.
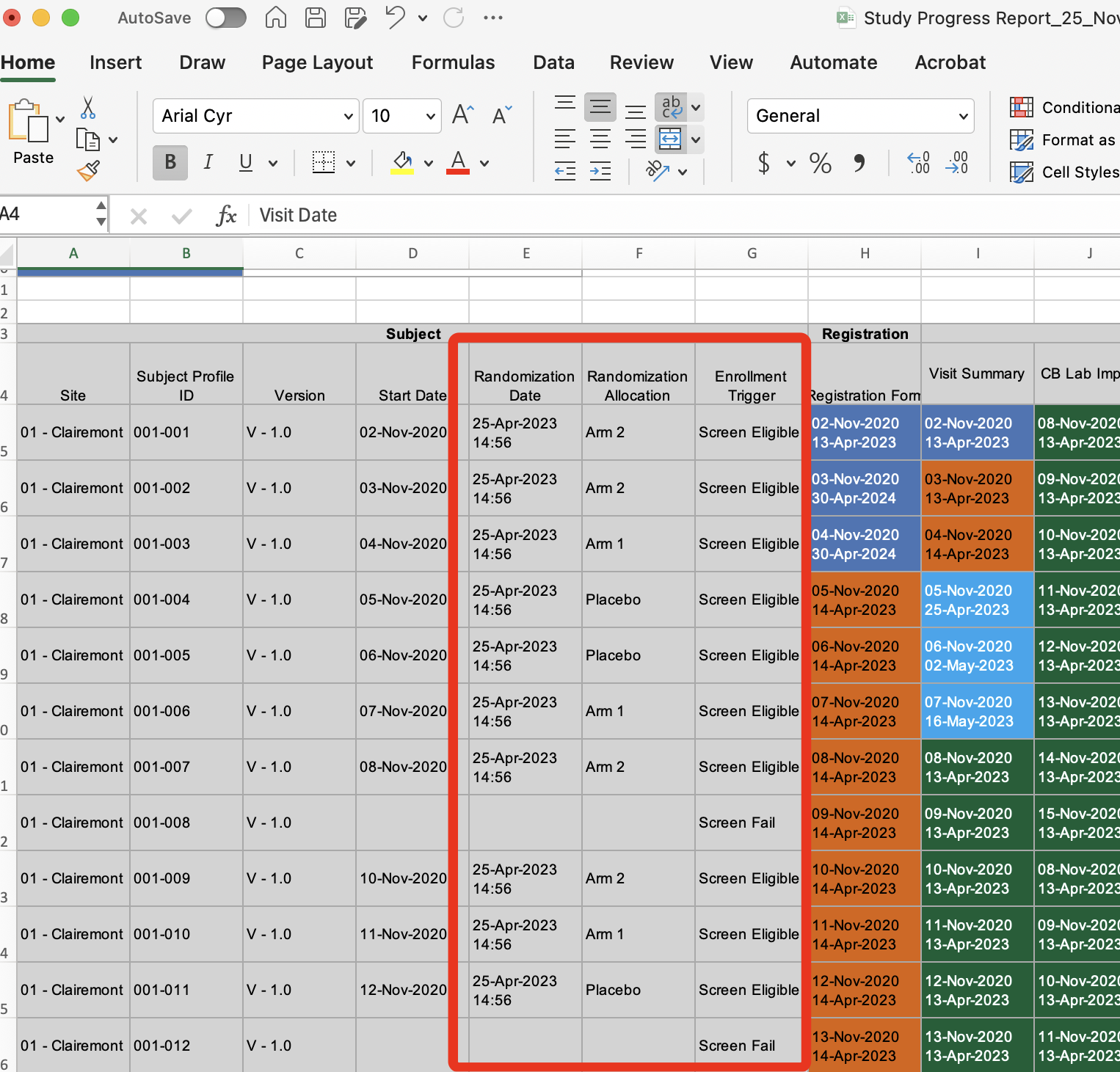
More Information In the Study Progress Report
The study progress report now contains the study’s Enrollment variable for each subject, so it’s easy to distinguish screen fails if applicable. It also now includes randomization allocation to unblinded users.
Other Helpful Mentions 😎
Additional key variables can now be defined for improved enrollment tracking.
Next generation forms now load 2x faster
Subject Progress tracker now accounts for conditionally hidden forms
Trial master file now allows reviewers to be named on placeholder. The reviewer will only get notified on the new task once the file has been uploaded.
API hooks can now return a customized message in the form save dialog popup window when users save forms
Copying and pasting external text into the form builder on the web now strips formatting to prevent bad HTML being brought into a form.
The report builder now includes the new e-signature data as option to include in reports
Participants New Features
Participant Access to Diary Schedules and Expired Diaries
Participants can now view their expected diary schedule(s). With permissions, they can even tap on past/missed diaries to enter them.
Participant Access to Their Own Data Listings
If a report has been made available to the Participants’ role in the report builder, they can access their
TrialKit Coder
Manual Coding Auto-add to the synonym list
When a term is manually coded, a prompt will be displayed to add it to the synonym list. This prevents the same term from needing to be be manually coded again.
Specific Coding Project Access
Access to specific projects can be controlled by form permissions in role security settings. Soon this will also be controlled directly in Coder.
Codes and Terms Split in the Exported Data
Codes and terms into separate columns for easier analysis on both WHODrug and MedDRA exports.
Download the full release notes ⬇️
Impact Considerations
See the release notes for impact of each change. Here are a couple that we anticipate some users will notice.
Data exports now contain a new column, “rel_log_trans_id”
Ancillary Form tools have been relocated from the top of the form to a new banner along the right side of the form
PDF exports from selected areas now display form and page name in the footer instead of the header (see above)
Coder Export File structure will split some of the data into more granular columns.
Upcoming Downtime Notice
End-of-year server upgrades and security patching will occur during non-peak hours on December 28 and 29, 2024. That update will include up to 24 hours of downtime. Notification will be getting sent to all account Administrators with more information.


